Case Study
Partnering With Visionix
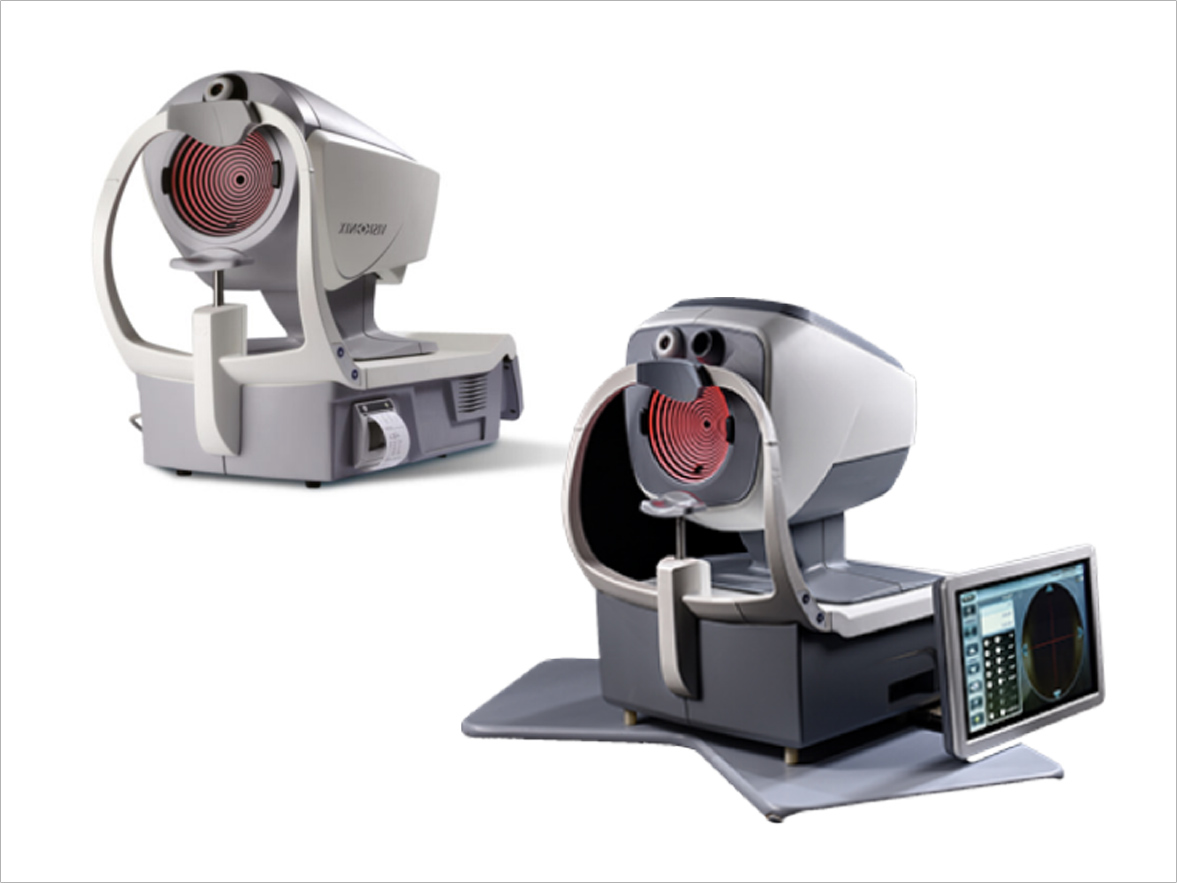
Visionix is a 20-year-old French organization in ophthalmic diagnostics, presented with a need to develop a comprehensive ophthalmic screening device for screening the anterior and posterior of the eye.
Tridios in-house R&D team developed an “automated easily retrofitted imaging module” currently integrated with VX-650+, a multi-modal device that conducts 24 eye exams in 90 seconds along with Retina!
Clinical and regulatory testing was performed as per EMC/EMI/ IEC and other regulatory compliances
Product stands currently approved and has the FDA 510(K) and CE mark
Continuous manufacturing and supply underway for these modules for VX-650+
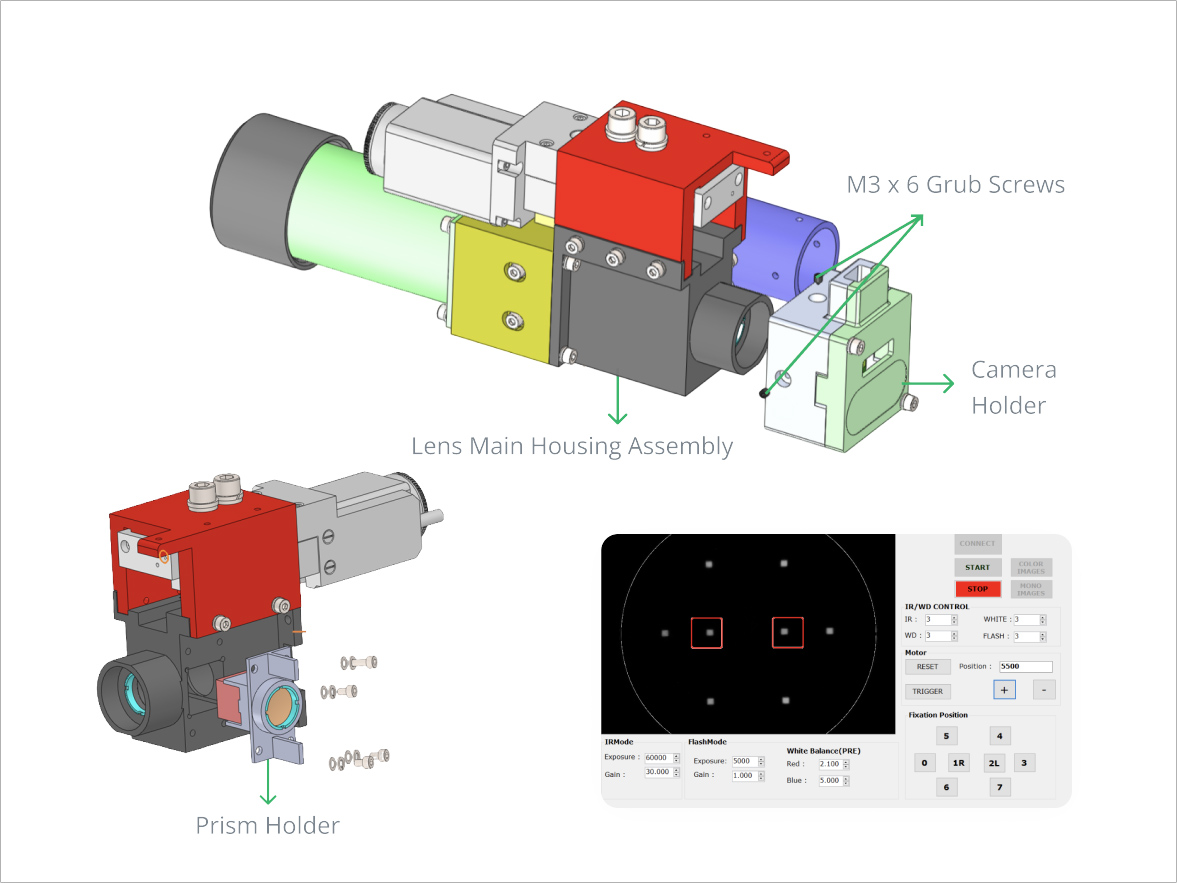
Third-Party OEM Manufacturing
Key Features
Integrated completely automatic system
Integrated camera/Autofocus and Auto-capture feedback mechanism
Regulatory approval (EMC, EMI, Safety….)
Biocompatible medical grade plastics and rubber material utilization
Inbuilt LCD Display system Party OEM Manufacturing
Processes Utilized
CNC machining and anodizing
UL/ROHS Compliant and traceable EMS for PCB boards
Biocompatible Plastic and rubber parts through Mould and 3D printing.
ISO Class 8 Assembly line and QA/QC as per 13485
Partnering For Respiraid
Key Features
Automated Respiration Assist device
Motors precision control system, Volume output
Regulatory approval (EMC, EMI, Safety, ISO-80601-2-84/ 80601-2-12 Safety Standard compliance….)
Biocompatible medical grade plastics and rubber material utilization
Inbuilt LCD Display system
Processes Utilized
CNC machining and Sheet metal parts
UL/ROHS Compliant and traceable EMS for complex PCB boards
Traceable control and volume measurement systems
Biocompatible Plastic and rubber parts through Mould and 3D printing.
ISO Class 8 Assembly line and QA/QC as per 13485



